Introduction: Iptacopan is a first-in-class, oral, selective inhibitor of factor B, a key component of the complement system alternative pathway. Inhibition of the complement system represents a potential mechanism for treatment of several diseases including paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. At the current clinical dose of 200 mg twice daily, the steady-state area under the concentration curve (AUC) and peak concentration (C max) are 25,600 ng•hr/L and 4,120 ng/mL, respectively. In this randomized, participant-blinded, placebo-controlled phase 1 study, we evaluated the pharmacokinetics (PK) of single high doses of iptacopan.
Methods: Iptacopan single doses of 400 mg (n=6), 800 mg (n=9), and 1200 mg (n=9) were included in this study, representing ≤6-fold higher doses than the clinical dose of 200 mg twice daily. The study population included healthy men and women aged 18-55 years. The log-transformed PK parameters AUC and C max were analyzed separately using a linear model with treatment as fixed effect.
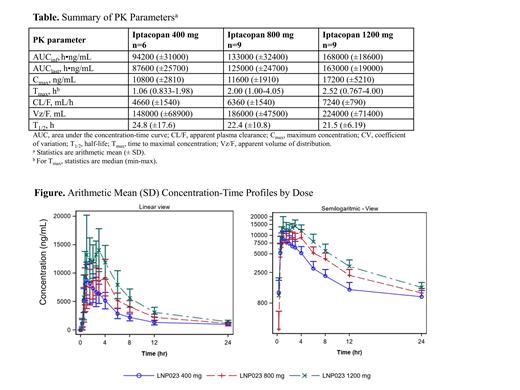
Results: Twenty-four patients received iptacopan and 8 received placebo. The median age of participants in the 400-, 800-, and 1200-mg groups was 51.5, 35, and 36 years, respectively. Following a single oral administration, iptacopan was rapidly absorbed with C max at 1.06, 2.00, and 2.52 hours for 400-mg, 800-mg, and 1200-mg doses, respectively ( Table). Variability (CV% geometric-mean) in iptacopan plasma concentrations were low to moderate (16.6%-30.6%) at C max. After administration, iptacopan plasma concentration-time profiles increased over the dose range of 400 to 1200 mg and were biphasic, with an inflection point at approximately 12 hours ( Figure). Compared to the 200-mg twice daily steady-state PK, the 1200-mg single dose resulted in C max and AUC exposure multiples of 4.0- and 6.5-fold, respectively. The mean apparent elimination half-life was similar across all doses, ranging from 20 to 21 hours. Iptacopan was well tolerated with no moderate or severe treatment-emergent adverse events reported.
Conclusion: Iptacopan was rapidly absorbed after administration of single oral doses ranging from 400 to 1200 mg. Variability in iptacopan plasma concentrations were low to moderate over the dose range studied and half-lives were similar. Supratherapeutic, single doses up to 1200 mg were well tolerated and resulted in substantial multiples of iptacopan systemic exposure.
Disclosures
Schmouder:Novartis Pharmaceuticals Corporation: Current Employment, Current holder of stock options in a privately-held company. Kaetterer:Novartis Pharma AG: Current Employment. Kulmatycki:Novartis Institutes of Biomedical Research: Current Employment. Nidamarthy:Novartis Healthcare Pvt Ltd: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal